INTRODUCTION
There are several reports of clinical and experimental studies emphasizing the role of left ventricular (LV) torsion in LV ejection (emptying) and suction (filling) as a sensitive indicator of LV performance [1-3]. In systole, from the apical perspective, the LV base twists clockwise while the LV apex twists counterclockwise, generating torsional deformations resulting in the dynamic interaction of oppositely wound epicardial and endocardial muscle fiber helices [4-6]. It is not clear if the right ventricle (RV) undergoes a similar twisting/untwisting motion of the myocardium, nor is the mechanism of the RV systolic torsional deformation or the impact on it of LV twist well understood.
The present study was designed to assess the global and segmental torsional motion of the RV in a normal population.
MATERIALS AND METHODS
Study design
We prospectively included as subjects either volunteers or patients with a normal sinus rhythm who underwent a routine echocardiographic exam. We excluded subjects with any history of hypertension, diabetes, ischemic heart disease, valvular heart disease, or routine echocardiographic findings of LV dysfunction as an ejection fraction < 50%, any regional wall motion abnormality, LV wall thickness ≥ 11 mm, diastolic dysfunction grade ≥ II [7], or a tricuspid regurgitation peak velocity ≥ 3.0 m/sec. We divided subjects with normal diastolic function (group I) and grade I diastolic dysfunction without elevated filling pressure (group II). This study was approved by an Institutional Review Committee and the subjects gave informed consent. The research was conducted in accordance with the principles of the Declaration of Helsinki.
Echocardiographic acquisitions and data analysis
We used a conventional 2D probe (M3S) with the Vivid 7 ultrasound system (GE Healthcare, Chicago, IL, USA), acquiring parasternal short-axis views of the RV at the basal and apical levels. We adjusted optimal 2D echo speckle imaging via changing transducer frequencies (1.7 to 2.0 MHz), frame rates (> 70 frames per second), and sector width (as narrow as possible to include both RV and LV), without dual-focusing.
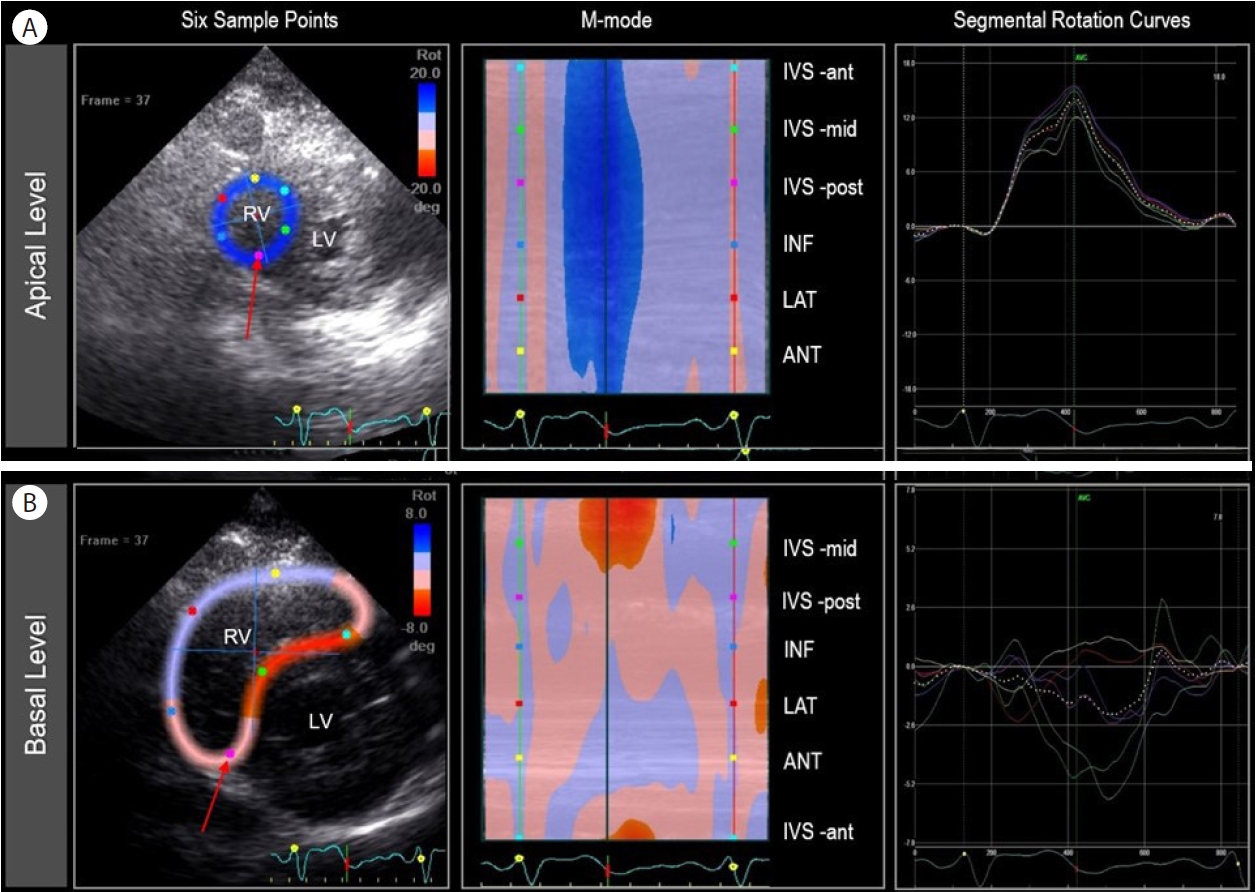
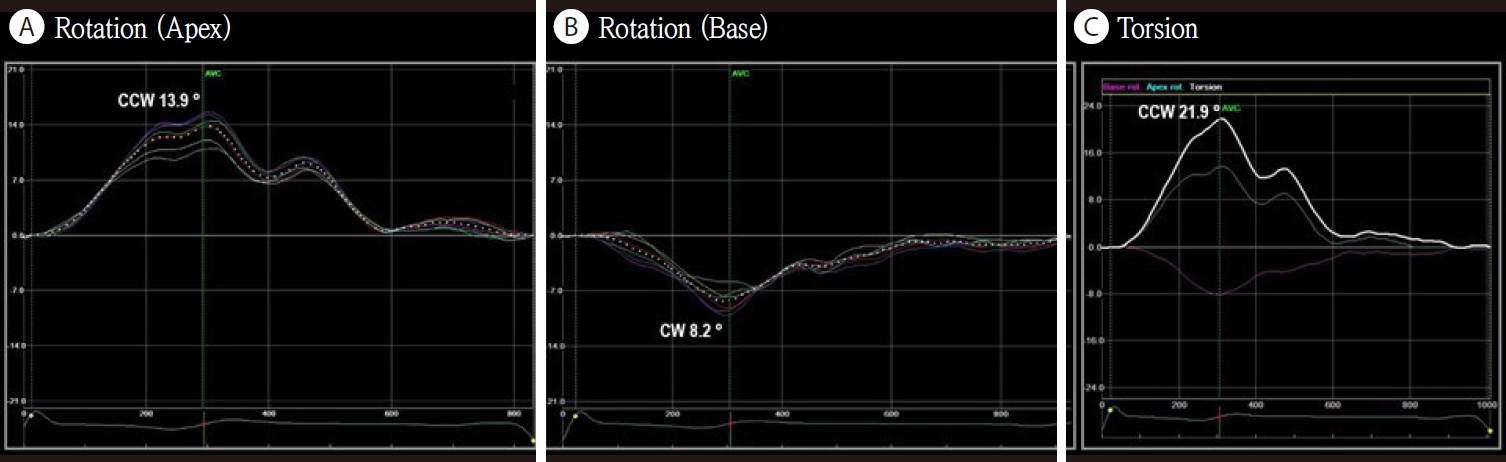
For 2D echo speckle-tracking data analysis, we used the EchoPAC Workstation (GE Healthcare, Wauwatosa, WI, USA) [8]. The RV and LV systolic ejection periods were determined from pulsed-wave Doppler imaging at the RV and LV outflow tracts, respectively. With conventional grayscale 2D parasternal short-axis views of the RV (basal and apical levels), semiautomatic speckle-tracking succeeded using a manual tracing of the RV endocardial border at end-systole with the smallest region of interest (ROI) width possible. Too narrow an ROI width results in poor tracking due to lack of tissue data in the ROI; too wide an ROI, due to static clutter. After inspecting each of the 6 segments and ensuring that the centerline is moving together with the underlying 2D image, we approved the 6 segments on the scoring table. From the results of semiautomatic tracking of the entire cardiac cycle, we analyzed both global (a dotted line) and individual segmental (six-solid lines) torsion curves after adjusting the bullet points (Fig. 1). A positive direction implies counterclockwise rotation (from the RV apical perspective), and a negative direction implies a clockwise rotation. We recorded the global and each segmental peak systolic rotation in degrees. Visually, the global torsion screen is available only when the apical and basal short axes have been processed. RV torsion was defined as the instantaneous net difference of the basal and apical rotation. We calculated the global and segmental RV torsion by subtracting the global or segmental rotation degrees from apex to base (Fig. 2).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. All statistical analysis was performed using IBM SPSS Statistics (version 16.0; SPSS Inc., Chicago, IL, USA). We assessed the correlation between continuous variables using Pearson’s correlation coefficient, and it was interpreted as negligible (< 0.1), weak (0.1-0.39), moderate (0.40-0.69), strong (0.70-0.89), or very strong (≥ 0.9) based on the absolute magnitude [9]. To compare continuous variables between the two groups, we used the independent-samples T-test; to compare continuous variables within the same patient, we used the paired-samples T-test. Two-sided p-values < 0.05 were considered statistically significant.
RESULTS
A total of 48 subjects were enrolled, 26 (54%) were male. The mean age was 44 years (range, 18-77). Analysis of RV rotation with the parasternal short-axis views using the 2D speckle-tracking algorithm was feasible in all study subjects who had adequate 2D gray-scale images. Normal diastolic function (group I) was seen in 40 patients, and grade I diastolic dysfunction without elevated filling pressure (group II) in eight patients.
Global RV torsion
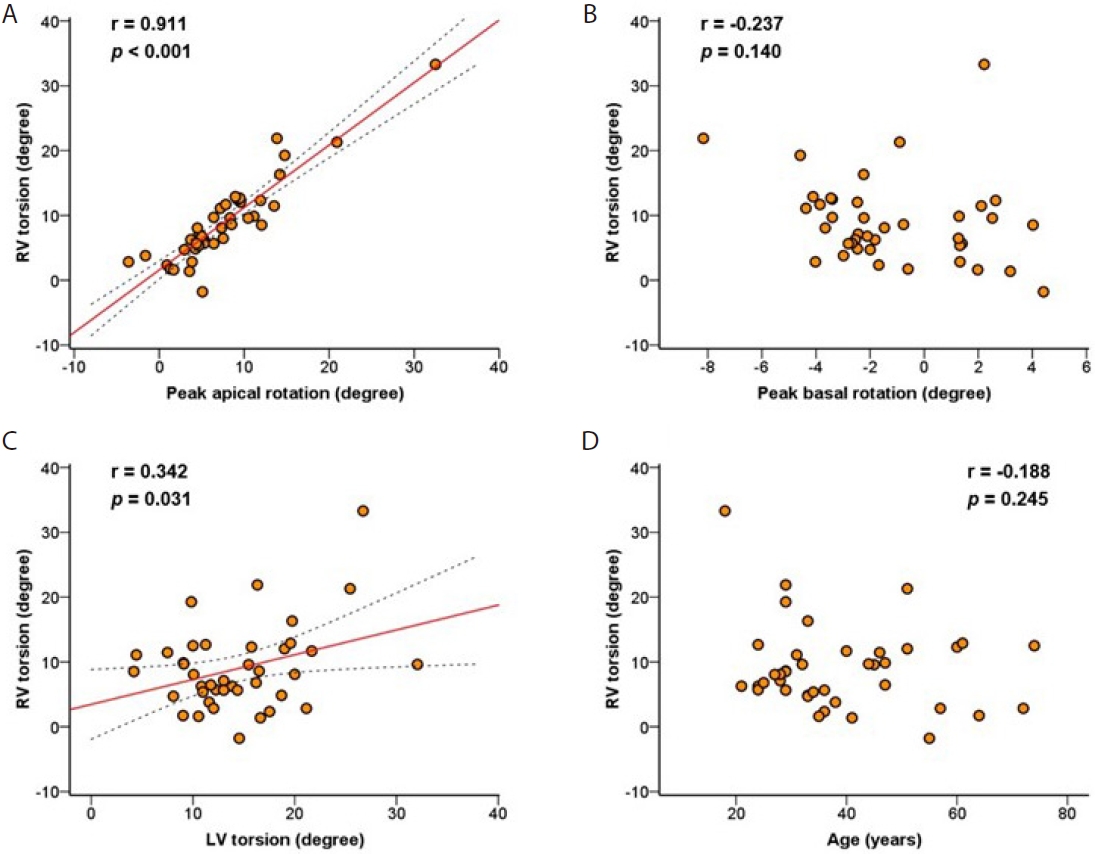
In subjects with normal diastology (group I), the peak apical rotation of the RV showed a counterclockwise rotation (7.7 ± 6.2 degrees); peak basal, clockwise rotation (-1.2 ± 2.8 degrees), and subsequent global RV torsion was a counterclockwise rotation (7.7 ± 7.4 degrees). The global torsional deformation showed a very strong positive correlation with the apical rotation (r = 0.911, p = 0.001), but not with the basal rotation (r = -0.237, p = 0.14) (Fig. 3). There was a weak positive correlation between the RV and LV torsion from the same apical and basal images (r = 0.342, p = 0.031) (Fig. 3).
Segmental RV torsion
Six-segmental rotation and torsional deformations of the RV are presented in Table 1. In subjects with normal diastolic function (group I), the peak apical counterclockwise rotation of the RV was high in the mid-to-posterior interventricular septum (IVS) and inferior walls compared with that of the anterior to anterior-IVS walls (p = 0.05), whereas the peak basal clockwise rotation was high in the anterior-IVS compared to the other walls (p = 0.05). The segmental torsion revealed a higher value in mid-IVS than in anterior walls (p = 0.001).
Comparison
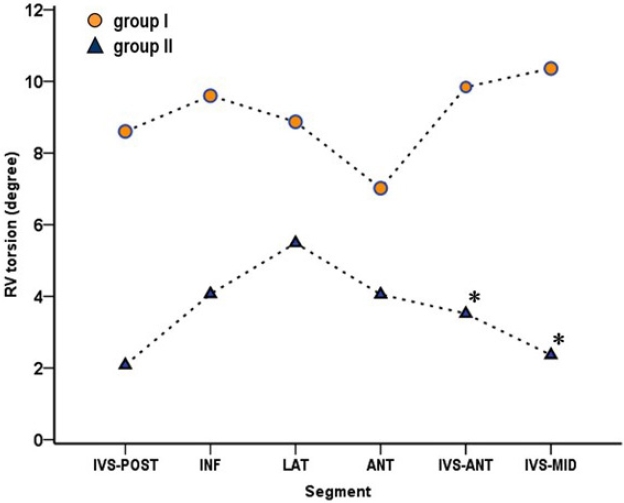
Subjects with normal diastolic function (group I, n = 40) were substantially younger than those with grade I diastolic dysfunction (group II, n = 8). Group I had significantly higher global as well as segmental RV torsions in anterior-IVS and mid-IVS segments than group II (9.0 ± 6.6 vs. 3.4 ± 9.2 degrees, p = 0.045; 9.8 ± 7.1 vs. 3.5 ± 9.6 degrees, p = 0.036; 10.4 ± 9.1 vs. 2.4 ± 12.3 degrees, p = 0.038, respectively) (Table 1, Fig. 4).
DISCUSSION
Analysis of RV global or segmental torsion was feasible using the 2D echocardiography speckle-tracking algorithm in all study subjects with adequate 2D gray-scale images. RV global torsion demonstrated a very strong positive correlation with RV peak apical rotation. The mid-IVS segment showed higher segmental torsion than the anterior segment of the RV in normal subjects.
Since 2005, novel ultrasound speckle-tracking imaging has been validated in humans with magnetic resonance imaging tagging [10,11] and clinically applied to assess LV rotation and torsion [1-3]. Using a 2D speckle-tracking algorithm, mean LV torsional deformation in a healthy adult population has been reported as a range of 10.0-14.2 degrees, increasing progressively until adulthood [12]. Combined with LV pump failure, reduced LV torsional deformation has been reported in various disease states [3,13]. However, only a few studies reported on RV torsion. Pettersen et al. [14] studied 14 patients with Senning operations to reveal the absence of the systemic RV torsion (0.3 ± 1.8 degrees) in contrast to normal LV torsion. Alizadehasl et al. [15] demonstrated a close correlation between RV and LV apical rotation in different cardiac conditions using the velocity vector imaging method. Motoji et al. [16] measured the apical longitudinal rotation at the peak rotation in the apical region including both LV and RV. To our knowledge, this is the first report to explore segmental RV torsion, which may benefit from overcoming echo drop-out in the right ventricular outflow tract (RVOT) at the basal RV level. Similarly, by avoiding the basal segmental RVOT dropout, apical rotation can be used as a simplified index of global RV torsion based on our correlation analysis (r = 0.911, p = 0.001).
The mechanism of RV torsion is not clear. From an anatomical point of view, we could consider two structures: the pericardium and the anisotropic biventricular muscle band. There was a report that LV torsion was unaffected by pericardiotomy [10], but patients with a congenital absence of the pericardium revealed a lack of LV torsion while maintaining LV strain [17]. There has been no report regarding any contribution of the pericardium to RV torsion. Anecdotally, we calculated RV torsion in a case of congenital total absence of the pericardium (Supplementary Fig. 1): the apical rotation was one standard deviation lower than that in normal subjects, hinting that the pericardium may indeed play a role in RV torsion. The biventricular, rope-like myocardial band also plays an important role in torsion. In the LV, a left-handed helix in the subepicardium and a right-handed helix in the subendocardium cause the LV apex to twist counterclockwise and the base clockwise from the apical perspective [4-6]. In the RV, the anisotropic morphology is more complicated. A subepicardial layer of myocardial fibers descends from the posterior basal RV edge to the anterior septum near the apex and enters into the septum in the anterior interventricular groove and ascends to the pulmonary artery root. Part of this superficial fiber traverses the septum from the anterior to the posterior interventricular groove and merges into the subendocardial layers. A subendocardial fiber ascends from the dorsal RV apex over the lateral mid-zone to the ventral base of the RV. A more apical portion of the subendocardial fiber ascends from the dorsal to the anterior RV wall and enters the septum [18,19]. In this study, global RV torsion was counterclockwise, to a smaller degree but in the same direction as the LV (Fig. 3C) despite the shared septal wall (by which the LV might be thought to induce RV counter-rotation); this may be the result of a joint-ventricular counterclockwise rolling motion.
An interesting finding in this study was the decrease of counterclockwise torsion in the RV IVS in patients with grade I diastolic dysfunction (Fig. 4). It has been reported that LV systolic torsion was significantly increased in patients with grade I diastolic dysfunction with a normal LV ejection fraction [8]. We speculate that with this increased counterclockwise rotation of the LV, the adjacent interventricular RV segmental torsion diminished its counterclockwise rotation; if validated, this implies a torsional interventricular dependency. The mechanical coupling of the anatomically conjoined ventricles [20] is supported in the three-dimensional muscular architecture [4,5,21]. Through the IVS, LV contraction contributes to RV contraction, similarly, LV torsion likely contributes to the RV interventricular segmental torsion as this study has shown.
We measured RV torsion from two parallel planes perpendicular to the RV long-axis. However, due to the complicated oblique muscle fibers across the RV, one might prefer a different axis to evaluate maximum torsion. Ultimately, three-dimensional RV imaging is needed to better understand RV torsional motion [22].
Analysis of RV rotation with the parasternal short-axis views using the 2D speckle-tracking algorithm was feasible. Segmental torsional analysis has the potential advantage of avoiding the echo drop-out in the RVOT. Apical rotation can be used as a simplified index of global RV torsion.